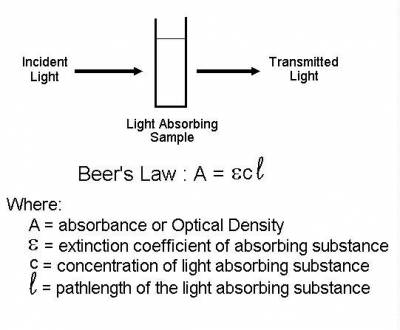
Turbidity or optical density is used in labs which measures the amount of light that is absorbed by the cellular content in a cuvette. It can be used to measure cell growth because the more light that is absorbed the more dense the cellular material. Turbidity can be measured by a spectrophotometer or a colorimeter. These devices measure the wavelength of the light absorbed through the cuvette. The absorbance is related to beer's law of A=εcl where A is absorbance, ε is the extinction coefficient , c is the concentration in Molarity and L is the length that the light passes through the cuvette which is measured in centimeters. The extinction coefficient can be calculated by determining the number of tryptophan, tyrosine, and cystine residues in the protein at absorption of 280 nanometers. The amino acid absorpbtion at 280nm of the three amino acids are: Tryptophan would be 5500, Tyrosine would be 1490 and Cysteine would be 125. So the formula would like:
ε= (nW×5500) + (nY×1490) + (nC×125)
Where W is Tryptophan, Y is Tyrosine and C is Cystine and n is the number the amino acid occurs in the protein.
|